How we enabled Visby Medical to go-to-market with their new PCR Covid-19 device, in just 8 weeks
Fast track sales force automation, customer service, and business process integrations to various systems for Visby Medical to market launch their FDA-approved Covid-19 PCR testing device.

Visby Medical, a new kind of diagnostics company, who are driven by a belief that disease diagnoses should be quick, accurate, and accessible to all, have developed a first-of-its-kind portable Covid-19 test kit. Accellor was entrusted with the task to assist in the go-to-market launch of this newly developed single-use Personal PCR. Visby Medical PCR provides a single-use test that delivers a fast and accurate result at the point of use.

Challenge
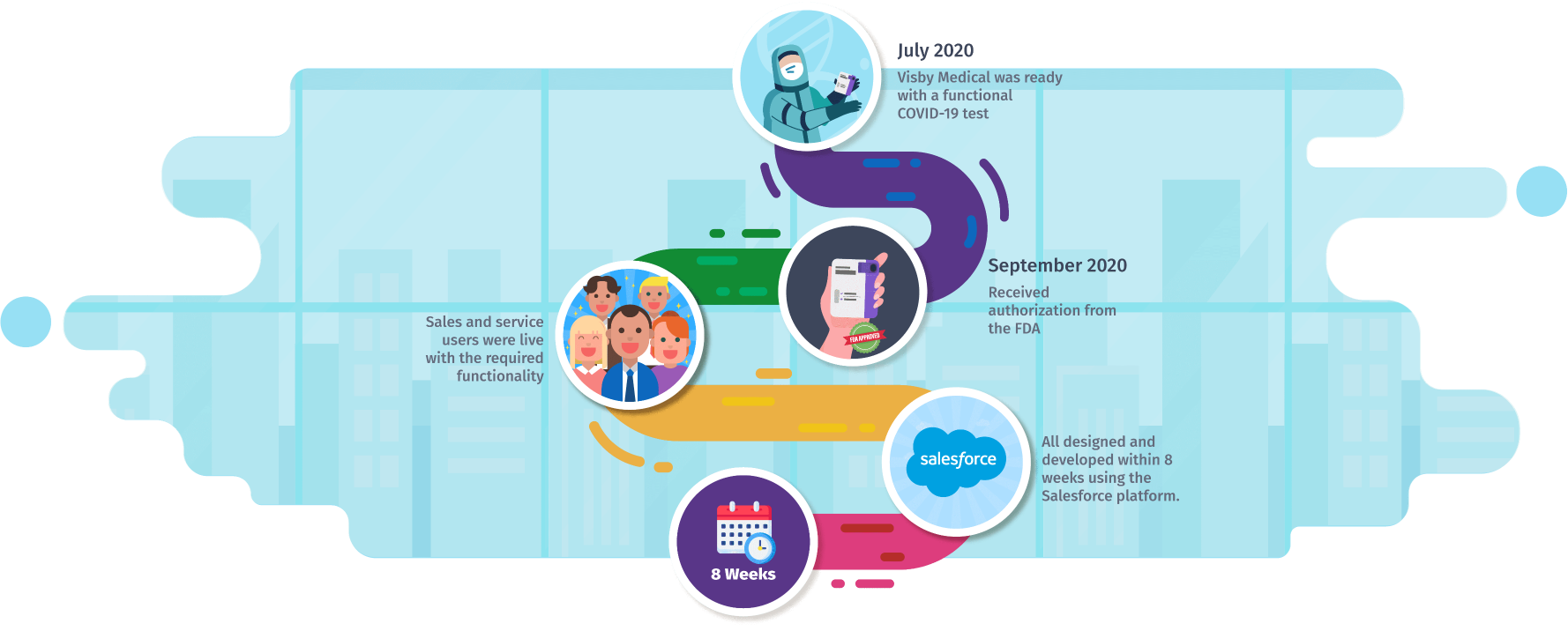
To get Visby’s Sales, Service, and integrated system up and running in a short period. In July 2020, Visby Medical was ready with a functional COVID-19 test and it was crucial to have processes and systems in place for business enablement in anticipation of the FDA approval. It received authorization from the FDA in September 2020. Sales and service users were live with the required functionality – all designed and developed within 8 weeks using the Salesforce platform.
Solution
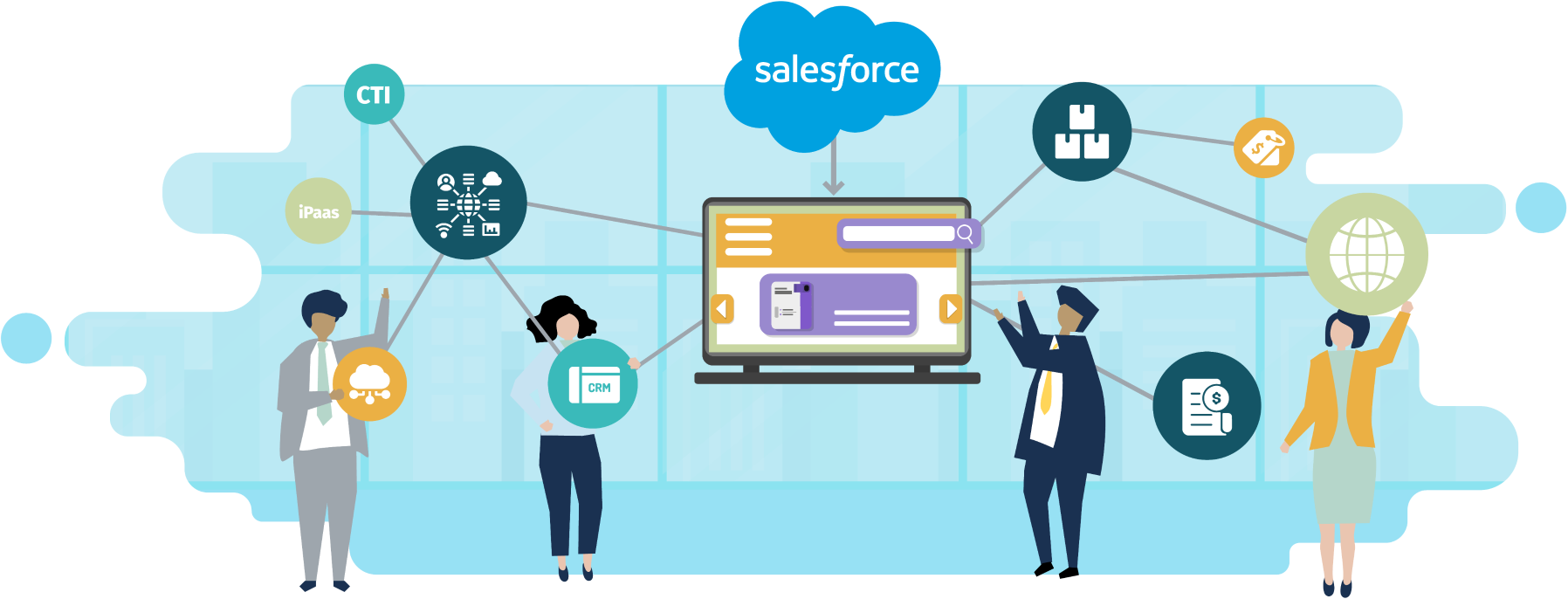
Sales personnel at Visby Medical can track leads, opportunities, quotes, and orders. Computer telephony integration using Zoom streamlines inbound call management and lead tracking. The implementation includes the first phase of integration to NetSuite which serves as the ERP and manages product pricing, inventory, invoicing, and fulfillment. Customers like hospitals and healthcare providers can also receive post-sales service and support through the enablement of Salesforce Service Cloud and the case management process.
Benefits
- Enabling sales & support processes to support the launch of Visby’s COVID PCR test kit | Personal PCR in your palm.
- End-to-end processes are smooth and the important pillars which are Sales, Customer Support, and Marketing are now seamlessly functioning.